Information in English
Nye metoder (“New methods”) is the national system of managed introduction of new methods in the specialist health care service in Norway. The system was launched in 2013.
The Norwegian health care service consists of the primary health care service and the specialist health care service. General information in English about central health rights and services in Norway (helsenorge.no). Nye metoder was established to be applied to new health technologies entering the specialist health care service.
As a general rule, until a positive decision has been made by Beslutningsforum (Nye metoder's Decision Forum), new medicinal products and indications cannot be prescribed in the specialist health services. After an assessment has been commissioned by the Ordering Forum, the pharmaceutical may be used in accordance with the exemption scheme (information in Norwegian), provided that specific requirements are met.
Health technology developers seeking public funding of a new medicinal product/ indication, must submit a request for assessment. Developers of other health technologies may submit proposals. More information for suppliers can be found here: For suppliers – information in English.
The purposes of the system
The systematic use of health technology assessments (HTAs) to inform decision-making was the main ambition behind the establishment.
Health technology assessment (HTA) is a scientific evidence-based process that allows authorities to determine the relative effectiveness of new or existing health technologies. HTA focuses specifically on the added value of a health technology in comparison with other new or existing health technologies.
Presentation: "Nye metoder and priority setting in the spesialist health care service"
Here you can find a presentation i English originally held for students at OsloMet University:
Broad cooperation
The four regional health authorities have the responsibility for the system. Nye metoder is based on a broad cooperation between:
- The four regional health authorities (the South-Eastern Norway Regional Health Authority, the Western Norway Regional Health Authority, the Northern Norway Regional Health Authority and the Central Norway Regional Health Authority) including all the hospitals
- The Norwegian Hospital Procurement Trust (Sykehusinnkjøp.no)
- The Norwegian Institute of Public Health (fhi.no)
- The Norwegian Medicines Agency (slv.no)
- The Norwegian Directorate of Health (helsedirektoratet.no)
- The Norwegian Radiation Protection Authority (dsa.no)
In addition, two stakeholder groups (Referansegrupper) are established, consisting of representatives from patient organizations, industry, professional associations and universities to contribute to the development of the system. The continuous dialogue with the industry associations within the pharmaceutical, medical devices and laboratory fields is important.
The principal components of the system
The system has to levels; a national level where decisions based on HTAs are made by the four regional health authorities in concert, and a local/”hospital level” where decisions are made based on the mini-HTAs performed locally in a hospital. The type of method and its designed authorized area of use are guiding to the suitable level for assessment. Certain methods, such as drugs, shall always be processed through the system at the national level. Put simple, both levels are composed of the following principal components:
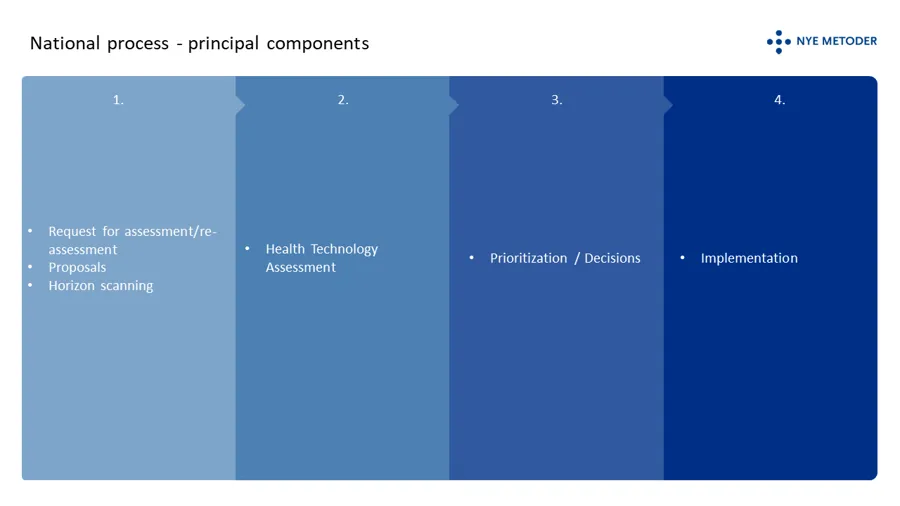
In order to be considered for public funding through the specialist health services in Norway, suppliers of a new medicinal product or a new indicationfor an existing medicinal product should request an assessment . It is open to everyone else to submit proposals fo health technology asssessments. Employees in the specialist health care can initiate a mini-HTA locally at their hospital.
Proposal form in English (Word)
An Ordering Forum, Bestillerforum, consisting of the four medical directors (one from each Regional Health Authority) and two delegates from the Norwegian Directorate of Health, has the mandate to prioritize the STAs and HTAs to be conducted on the basis of submitted requests of assessment, proposals and horizon scanning reports.
After completion of an STA, the Norwegian Hospital Procurement Trust (Sykehusinnkjøp HF) conducts negotiations. Subsequently a Decision Forum comprised of the four CEOs (one from each Regional Health Authority) makes a decision on whether to introduce the method or not.
Information for suppliers
Contact us
Further questions about the system can be directed to the Secretariat of Nye metoder: nyemetoder@helse-sorost.no